Abstract
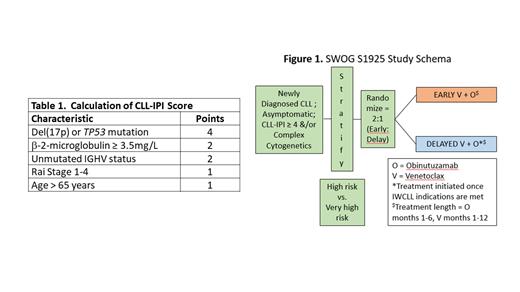
Background: Currently, asymptomatic patients with CLL/SLL are observed without treatment until development of symptoms or cytopenias. Historically, early intervention studies in patients with CLL/SLL with non-specific chemoimmunotherapy agents have not resulted in an overall survival (OS) benefit and have resulted in toxicity. The introduction of targeted therapies, such as venetoclax (an oral BCL2 inhibitor; V) and obinutuzumab (an intravenous anti-CD20 monoclonal antibody; O), have provided tolerable/efficacious options for patients with CLL. In the CLL14 study, symptomatic patients with CLL receiving frontline therapy with VO had longer progression-free survival (PFS) and deeper remissions [more undetectable minimal residual disease (uMRD)] compared with those receiving chlorambucil and O (Fischer 2019). The CLL-International Prognostic Index (CLL-IPI; Table 1) is a validated prognostic model to predict which patients are at highest risk of a shorter time to first therapy and shorter OS. A score of ≥4 is considered high-risk on this scale. We aim to use VO as early intervention in asymptomatic, high-risk CLL patients, assessed by CLL-IPI, to potentially improve OS and thus alter the natural history of the disease.
Methods: On 12/14/20, we activated the S1925 study (NCT#04269902 ) for adult patients with CLL or SLL, who were diagnosed within 12 months of enrollment. Eligible patients have a CLL-IPI score ≥4 (Table 1) or complex cytogenetics (≥3 cytogenetic abnormalities) and do not meet any criteria for initiation of treatment by the International Working Group for CLL (IWCLL; Hallek 2018) guidelines. Enrolled patients are randomized in a 2:1 manner to early versus delayed (at the time IWCLL indication for treatment is met) therapy with VO (Figure 1). VO is administered as previously described (Fischer 2019). The primary endpoint is OS. We hypothesize that early intervention with VO will improve the rate of 6-year OS from 60% to 80%. This design requires 222 eligible patients for 88% power (2-sided a=0.05) for the primary comparison. To allow for 10% ineligibility, we will enroll 247 patients. Estimated accrual time is 4 years. Secondary endpoints include: rates of response, PFS, and relapse-free survival; safety; time to second CLL-directed therapy; and quality of life (assessed by FACT-Leukemia). As COVID19 is an infection with particularly high morbidity and mortality in patients with CLL, incidence of this infection and complications including death will be recorded and compared between patients followed on the early versus delayed intervention arms. The primary translational objective is to evaluate the prognostic association between OS and peripheral blood MRD status at 15 months after treatment initiation by flow cytometry. Secondary translational objectives include describing the association of other clinical outcomes, baseline prognostic factors, and IWCLL-defined response with MRD status at multiple timepoints.
Current Status: At the time of submission, 7 patients have been registered and randomized per protocol. Accrual is ongoing.
Stephens: Adaptive: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Epizyme: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Innate Pharma: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy; CSL Behring: Consultancy; Celgene: Consultancy; Novartis: Research Funding; Abbvie: Consultancy; JUNO: Research Funding; Arqule: Research Funding; Mingsight: Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding. Moseley: BioSight Ltd: Consultancy. Hill: AbbVie: Consultancy, Honoraria, Research Funding; Gentenech: Consultancy, Honoraria, Research Funding; Beigene: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel Support, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding; AstraZenica: Consultancy, Honoraria; Celgene (BMS): Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Epizyme: Consultancy, Honoraria; Incyte/Morphysis: Consultancy, Honoraria, Research Funding. Pagel: Pharmacyclics/AbbVie: Consultancy; Actinium Pharmaceuticals: Consultancy; Incyte/MorphoSys: Consultancy; BeiGene: Consultancy; Epizyme: Consultancy; Kite, a Gilead Company: Consultancy; AstraZeneca: Consultancy; Gilead: Consultancy; MEI Pharma: Consultancy. Shadman: Mustang Bio, Celgene, Bristol Myers Squibb, Pharmacyclics, Gilead, Genentech, Abbvie, TG Therapeutics, Beigene, AstraZeneca, Sunesis, Atara Biotherapeutics, GenMab: Research Funding; Abbvie, Genentech, AstraZeneca, Sound Biologics, Pharmacyclics, Beigene, Bristol Myers Squibb, Morphosys, TG Therapeutics, Innate Pharma, Kite Pharma, Adaptive Biotechnologies, Epizyme, Eli Lilly, Adaptimmune , Mustang Bio and Atara Biotherapeutics: Consultancy. Danilov: Genentech: Consultancy, Honoraria, Research Funding; Takeda Oncology: Research Funding; TG Therapeutics: Consultancy, Research Funding; Abbvie: Consultancy, Honoraria; Beigene: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Gilead Sciences: Research Funding; Bristol-Meyers-Squibb: Honoraria, Research Funding; Rigel Pharm: Honoraria; Bayer Oncology: Consultancy, Honoraria, Research Funding; SecuraBio: Research Funding; Astra Zeneca: Consultancy, Honoraria, Research Funding. Mato: Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; DTRM BioPharma: Consultancy, Research Funding; Acerta/AstraZeneca: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Johnson and Johnson: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Nurix: Research Funding; Genmab: Research Funding; LOXO: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; AstraZeneca: Consultancy; Adaptive Biotechnologies: Consultancy, Research Funding; MSKCC: Current Employment; TG Therapeutics: Consultancy, Other: DSMB, Research Funding. Brander: Juno Therapeutics/Celgene/Bristol Myers Squibb: Research Funding; Pfizer: Consultancy, Other: Biosimilars outcomes research panel; TG Therapeutics: Consultancy, Research Funding; Novartis: Research Funding; ArQule/Merck: Consultancy; Verastem: Consultancy; BeiGene: Research Funding; ArQule: Research Funding; NCCN: Other: panel member; AstraZeneca: Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; LOXO: Research Funding; Ascentage: Research Funding; Genentech: Consultancy, Research Funding; DTRM: Research Funding; MEI Pharma: Research Funding; AbbVie: Consultancy, Other: informCLL registry steering committee, Research Funding. Coutre: Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Beigene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Data Safety Monitoring Committee, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Acerta: Other: Data Safety Monitoring Committee, Research Funding. O'Brien: Kite, Regeneron, Acerta, Caribou, Gilead, Pharmacyclics, TG Therapeutics, Pfizer, Sunesis: Research Funding; Amgen, Astellas, Celgene, GlaxoSmithKline, Janssen Oncology, Aptose Biosciences Inc., Vaniam Group LLC, AbbVie, Alexion, Verastem, Juno Therapeutics, Vida Ventures, Autolus, Johnson and Johnson, Merck, Bristol Myers Squibb, NOVA Research Company, Eli Lill: Consultancy. Erba: AbbVie Inc; Agios Pharmaceuticals Inc; Bristol Myers Squibb; Celgene, a Bristol Myers Squibb company; Incyte Corporation; Jazz Pharmaceuticals Inc; Novartis: Speakers Bureau; AbbVie Inc: Other: Independent review committee; AbbVie Inc; Agios Pharmaceuticals Inc; ALX Oncology; Amgen Inc; Daiichi Sankyo Inc; FORMA Therapeutics; Forty Seven Inc; Gilead Sciences Inc; GlycoMimetics Inc; ImmunoGen Inc; Jazz Pharmaceuticals Inc; MacroGenics Inc; Novartis; PTC Therapeutics: Research Funding; AbbVie Inc; Agios Pharmaceuticals Inc; Astellas; Bristol Myers Squibb; Celgene, a Bristol Myers Squibb company; Daiichi Sankyo Inc; Genentech, a member of the Roche Group; GlycoMimetics Inc; Incyte Corporation; Jazz Pharmaceuticals Inc; Kura Oncology; Nov: Other: Advisory Committee.
The trial studies early intervention with venetoclax and obinutuzumab in patients with CLL/SLL who are asymptomatic and observation would be standardly recommended.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal